2.1 Volumetric analysis
It really is best that you attempt as many problems as possible to revise for this subtopic. It is also worthwhile revising (and perhaps memorising if necessary) how to convert between different conversion units.
Some points to remember:
- don’t forget to convert mL to L
- write the correct number of significant figures for your responses (see below for more information) but ensure that you keep the unrounded number in your calculator for the whole question.
- C1V1 = C2V2 can be useful
Volumetric pipette
(note the use of the word VOLUMETRIC – it is important)
- designed to deliver a fixed volume of liquid accurately.

- washed with distilled water, allowed to drain and rinsed with solution to be pipetted before use. This will ensure that the concentration of the solution to be pipetted is not diluted by the distilled water.
- use a pipette filler to fill above the calibration mark.
- allow solution to drain until the bottom of the meniscus lies on the calibration mark (ensure no air bubbles present).
- touch tip to side of conical flask (previously washed with distilled water) and allow solution to drain.
- the pipette should be kept vertical and the tip should remain touched to the side of the flask for 10-15 seconds after the solution appears to have completely drained.
- the pipette is calibrated for the last drop to remain, so it should not be removed.

Burette
- designed to deliver variable amounts of liquid accurately.
- usually marked in 0.1mL divisions and can be read to 0.05mL.
- rinse with distilled water ensuring every part has been rinsed, drain and rinse with solution to be delivered (ensure some solution runs through the tap as well). This will ensure that the concentration of the solution the be delivered is not altered.
- fill with solution to be delivered. Ensure no gas bubbles are in the tap and that the funnel has been removed from the top.
- record initial volume.
- perform titration.
- record final volume and calculate total volume of solution delivered.
Volumetric flask
- designed to measure volumes of liquids accurately.
- rinse with distilled water before use.
- ensure bottom of meniscus is in line with the calibration mark once filled.
- INVERT BEFORE USE TO ENSURE THAT THE SOLUTION IS HOMOGENEOUS.
Conical flask
- wash with distilled water before use to ensure that contaminants are removed and that the number of moles of solution to be added is accurately known.
Endpoint
- the end point of a titration occurs when the solution in the conical flask first shows a permanent colour change.
- it is important to note that the end point is not the same as the equivalence point (the point at which the amount of titrant added is exactly enough to react completely with the compound being analysed). However, it is a very good approximation of the equivalence point and is assumed to be the same.
| Type of titration |
Indicator |
Colour change |
| Base added to strong acid |
Bromophenol blue |
Yellow to blue |
| Base added to weak acid |
Phenolphthalein |
Colourless to pink |
| Iodine solution added to solution of reducer |
Starch |
Colourless to blue |
| Permanganate solution added to a colourless solution of a reducer |
No indicator |
Colourless to pink |
Significant figures
Although you can get some easy marks if you do this correctly, in the grand scheme of things, not many marks are allocated for this….
- the final answer to any calculation must contain the same number of figures as the least precise piece of data given in the problem.
- the number of significant figures in a piece of data is determined by counting the number of digits from the first non-zero digit. For example; 0.200, 0.0200 and 0.00200 all contain 3 significant figures.
- you can read more and practise here.
I know that I’ve referred you to this website before, but it has animations and videos for volumetric analysis (as well as other relevant topics such as chromatography) that you might find helpful.
2.2 Chromatography
Chromatography is the process of separating small amounts of substances from mixtures by the relative speeds at which they move along a medium. There are many chromatographic techniques, but they all rely on the components of the mixture being carried by a mobile phase over a stationary phase at different rates.
To perform chromatography, mobile and stationary phases with very different polarities are used. this is because the mixture to be separated will contain components of varying polarities. As a result, each component will be attracted to the stationary and mobile phases to differing degrees. Those most strongly attracted to the mobile phase will move over the stationary phase at a greater rate than those more strongly attracted to the stationary phase. this means that each component will travel at a different rate to the others. Eventually all the components of the mixture will pass over the stationary phase. Each component can be identified by comparing its movement to a set of known standards.
Adsorption
- the attraction of molecules onto the surface of a solid, forming a thin film over the surface.

Normal phase vs reverse phase
- in normal phase chromatography the stationary phase is more polar than the mobile phase.
- in reverse phase chromatography the mobile phase is more polar than the stationary phase.
Thin layer chromatography (TLC)
- thin plate covered in silica or alumina is used as the stationary phase.
- the mobile phase must be a liquid.
- the following thin layer chromatography animation is a trial version, but gives you a bit of an idea how this works.
- although not interactive, the following sites provide a useful summary for thin layer chromatography and how it works (and it saves me from having to write it all again for you). Definitely worth reading.
Rf
- a measure of the relative speed of a component along the stationary phase.
- distance moved by a particular component divided by the distance moved by the solvent.
- take your measurement from the middle of the spot.
Column chromatography
- read about it here: column chromatography.
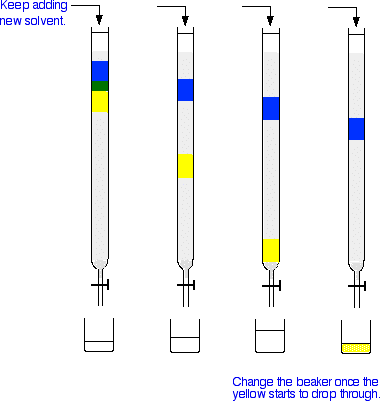
Gas-solid chromatography
- stationary phase consists of uniform size particles either packed in a long narrow tube or coated on the inside surface of the tube.
- sample injected and is then vaporised.
- movement of the vaporised mixture through the column is brought about by the flow of the mobile phase (commonly an inert gas such as helium or nitrogen).
- as the mixture is carried through the column, separation is achieved and the various components leave the column at different times.
- the more strongly a component attaches to the stationary phase, the longer it takes to exit the column.
- on leaving the column, each component passes through a detector that sends a signal to a recorder that produces a peak.
- the series of peaks representing all of the components of the mixture is called a chromatogram.
Retention time
- in some types of chromatography the factor used to compare the movement of the components of a mixture is the retention time.
- retention time is the time taken for a component to move through the chromatogram.
2.3 Atomic spectroscopy
Electrons occupy distinct energy levels within an atom. Each energy level corresponds to a particular potential energy. Therefore in order for electrons to move from one energy level to another, a discrete amount of energy must be gained or lost by an electron. This energy takes the form of electromagnetic radiation. When radiation is absorbed, electrons move to higher energy levels whilst radiation is emitted when electrons move to lower energy levels.
- movement of electrons from the ground state to excited states will produce an absorption spectrum
- movement of electrons from excited states to ground state will produce an emission spectrum
The wavelengths of radiation absorbed and emitted by an element are unique to that element due to the distinct energy levels that exist within each element.
Atomic absorption spectroscopy (AAS)
- each different element will absorb and emit unique wavelengths of radiation.
- this fact can be used to identify the elements present in an unknown sample using AAS.
- use a lamp containing the same metal as that being determined in the sample, eg, a sodium lamp is used to identify sodium
- light from this lamp is shone through a spray of the mixture being analysed, where only the atoms of the element in question will absorb light from the lamp.
- a monochromator selects one of the specific wavelengths of light after it has passed through the sample to the detector.
- the measurement is compared to measurements from samples of known concentrations of the particular element (calibration curve).

But more specifically, how do we obtain the calibration curve?
- prepare a set of standard solutions and analyse them with AAS
- the absorbance values from the analysis are plotted against the corresponding concentrations of the standard solutions.
- a line of best fit is added
- when analysing an unknown sample, the amount of light absorbed can be calculated as the intensity of a beam with no sample, minus the measured intensity of the sample present. This absorbance depends on the quantity of element present.
Some more revision that may be useful
pH
some useful equations:
- pH = -log[H+]
- pOH = -log[OH-]
- pH + pOH = 14
- [OH-] =10-pOH
- [H+] =10-pH
some further revision can be located here.
balancing redox equations:
Its late at night and I need to go to bed so you can locate some revision material for this here….
Happy revising!
Good luck!
Mrs Elliott